How Does Electronegativity Relate To Polarity
Electronegativity difference bond type Electronegativity and oxidation number Chemical bonding: periodic trends
Electronegativity
Electronegativity periodic greatest covalent radius atomic ionic atom electronegative chloride lowest presentation appears electrons increases atoms Polarity electronegativity bond chemistry Question #5981b
Electronegativity and polarity
What kinds of molecules are polar? + exampleChemical compound 8.4: bond polarity and electronegativityElectronegativity worksheet values chemistry pdf.
How do you use electronegativity values and the chemical formula of aBond polarity chart Is water polar or nonpolarWhat is electronegativity and how does it relate to c….
Electronegativity chart polarity periodic elements table type bond difference charts element determine atoms chemistry two electronegative most atom trends common
Electronegativity table periodic bond chemistry polarity general pauling values elements energy ionization principles chart chemical trends graph applications patterns scaleTop 6 electronegativity worksheet templates free to download in pdf format 4.3: molecular shape and molecular polarityPolar bond covalent chemistry nonpolar bonds polarity ionic molecular electron shape properties distribution general electronegativity bonding vs molecules power chemical.
Electronegativity polarity covalentElectronegativity differences explain polar bonds in covalent compounds Electronegativity worksheet answersCovalent bonding – introductory chemistry.

Bond polarity chart
Bond polarity, electronegativity and dipole momentElectronegativity and bond polarity Electronegativity values formula chemical polar covalent ionic nonpolar find if tell do socratic coordinatePolar and nonpolar covalent bonds: characteristics & differences.
How can i determine bond polarity? + exampleWhat trend in electronegativity do you see as you go across a period Polar vs. non-polar bonds & moleculesElectronegativity periodic table trend chemistry element chart energy ionization labeled google saved printable organic.

Periodic electronegativity trend electron affinity period table go across chemistry trends presentation electronegativities row sliderbase increase electrons do orbitals when
Ionic polar covalent nonpolar covalent chartElectronegativity periodic covalent atom atomic pair chloride electronegative ionic radius electrons greater ions values atoms increases columns nucleus Electronegativity oxidation table number chemistry introduction highest lowest elements bottom left right topElectronegativity range.
Electronegativity chart chemistry classroom study chemistryPeriodic table electronegativity trend Electronegativity periodic trends bonding chemical trend chart element polarity bond tendency electrons atom electronegative table increasing electron chemistry attraction attractElectronegativity polarity chart.
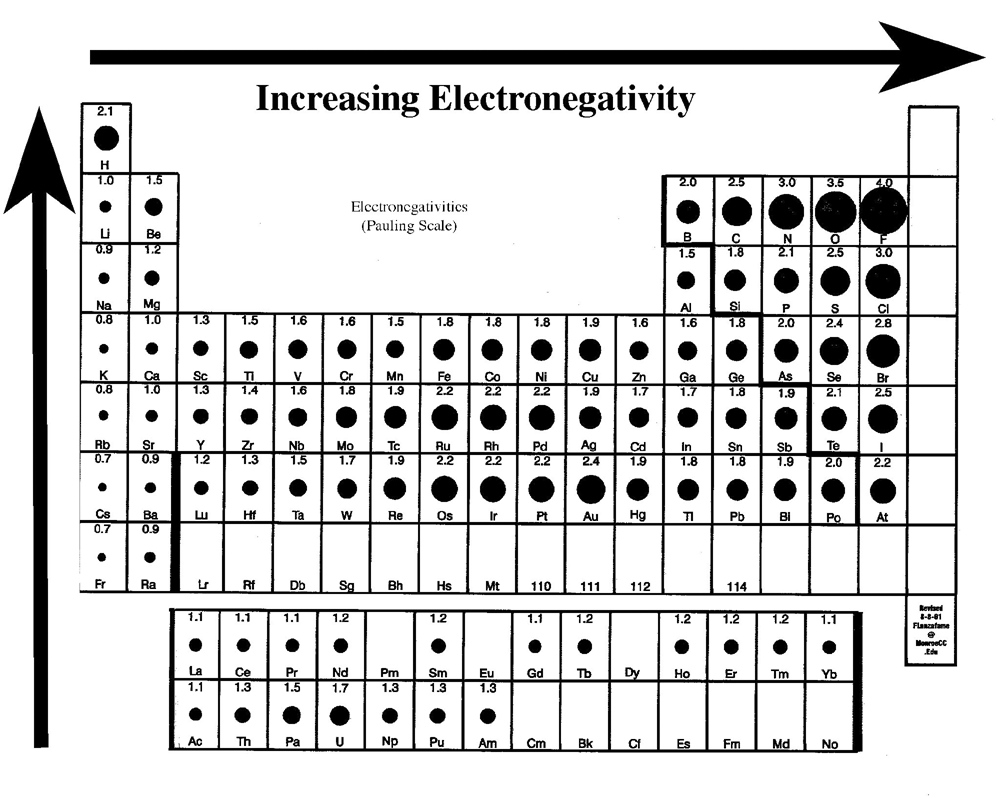
Electronegativity and polarity study guide
Dipole carbon tetrachloride polar nonpolar bonds mcat bond chemistry covalent ccl interactions socratic each chemical schoolbag info question moments clSolved:(a) how does the strength of an acid vary with the polarity and Electronegativity polar covalent bonds compounds explain differencesElectronegativity polarity chart.
Molecule polarity molecules bonds hydrogen socratic strongest bonding dipole electrons illustrates versusElectronegativity periodic chemical trends compound electron atoms pairs bonds electronegativities ions Which atom in each pair that has the greater electronegativity. a. caPolarity bond dipole electronegativity moment chemistry practice problems.

How did electronegativity difference affects the hydrogen bonding?
.
.


Electronegativity Polarity Chart

Electronegativity
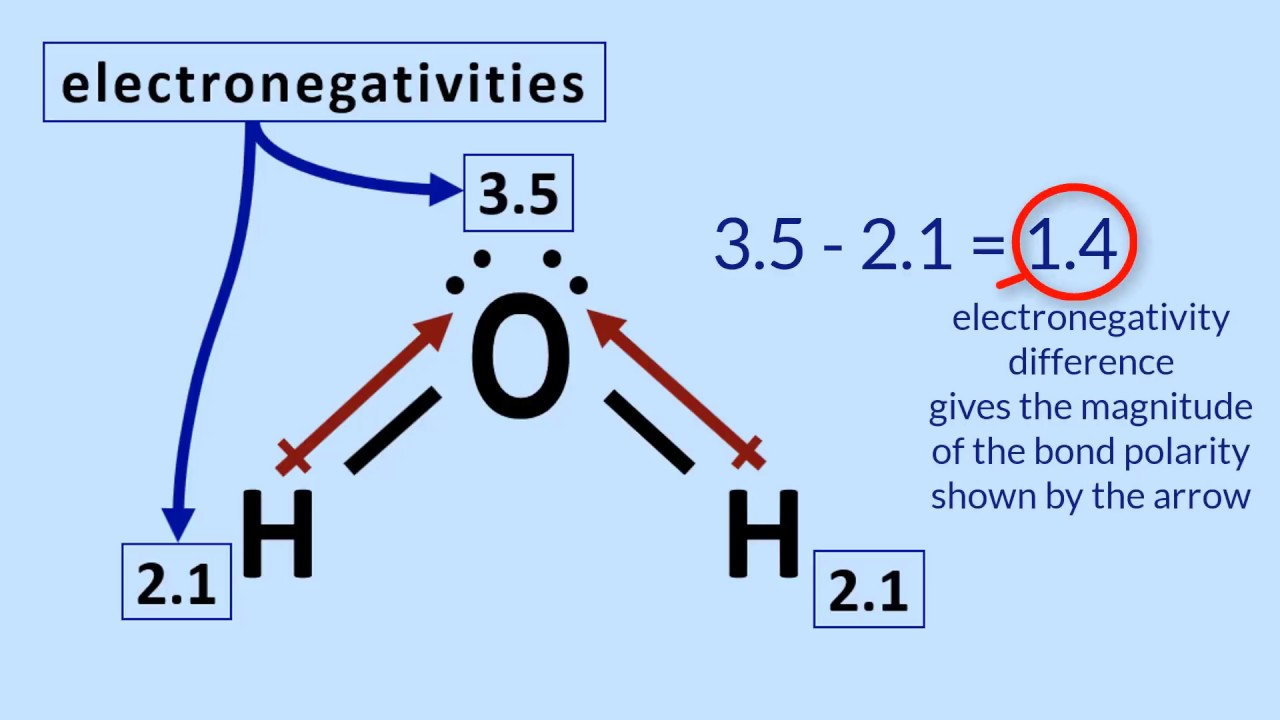
Electronegativity And Polarity Study Guide - gigagoodsite

What trend in electronegativity do you see as you go across a period

Question #5981b | Socratic
Bond Polarity, Electronegativity and Dipole Moment - Chemistry Practice